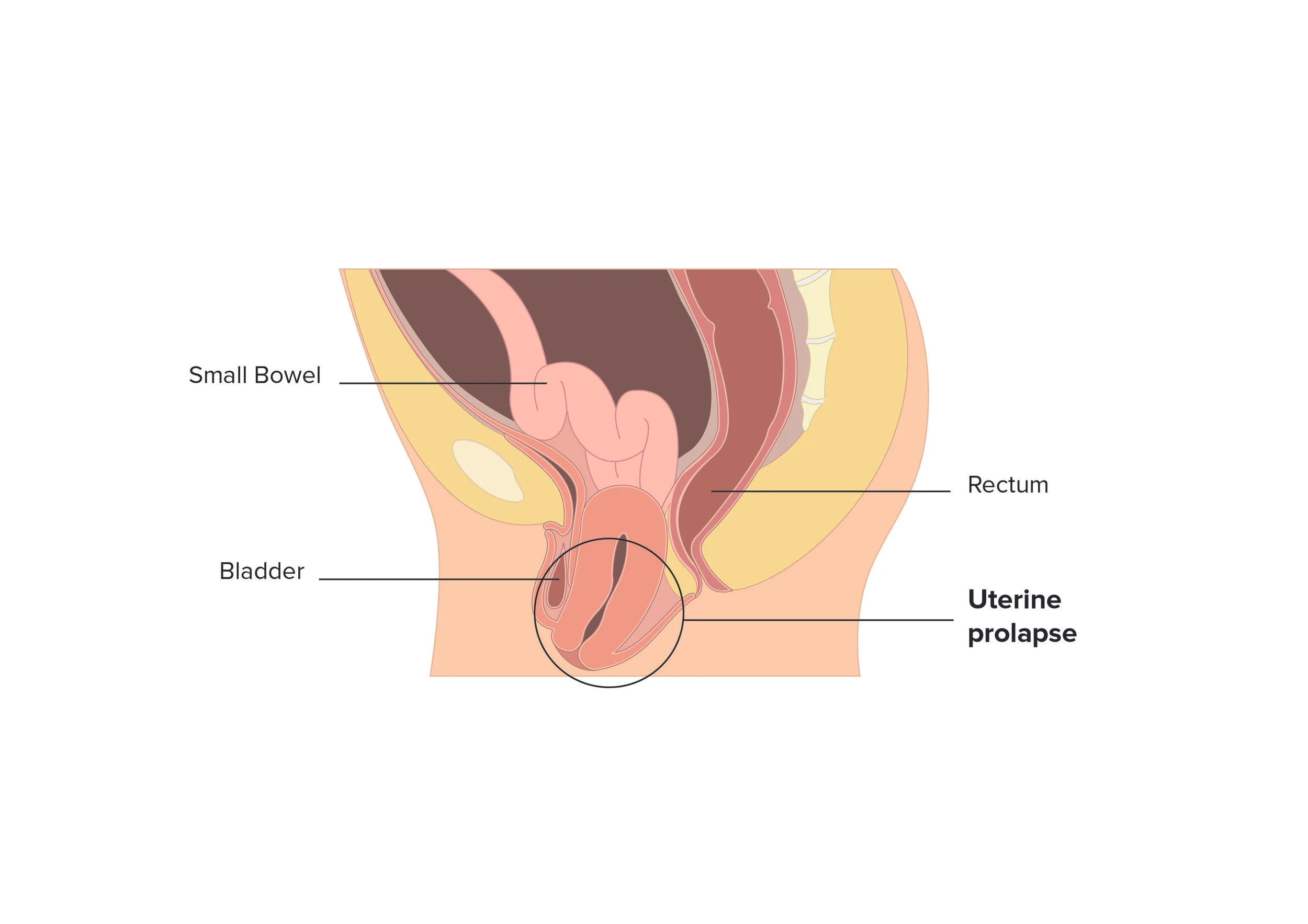
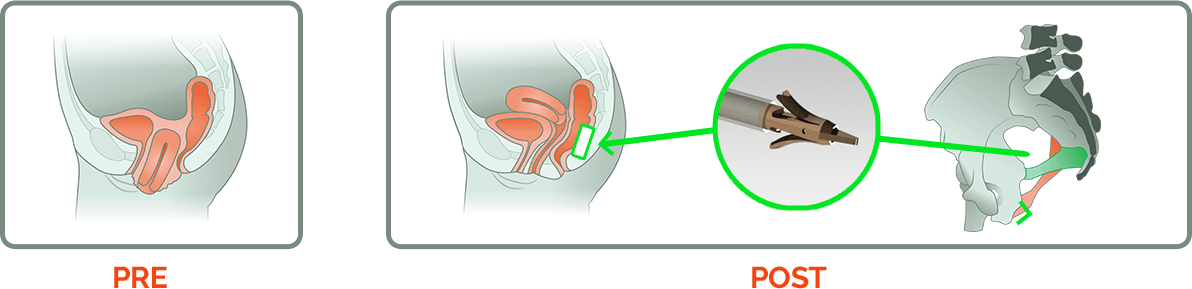
Uterine Prolapse (APOPS)
Global, systemic evolution of research and treatment for pelvic organ prolapse (POP) is not a maybe, it is a must, considering up to 50% of women are estimated to experience this condition. The needs of women vary based on type(s) of POP and country of residence. There is significant prevalence of uterine prolapse, both in developed zones such as the US, UK, Europe and Australia, as well as in developing zones such as Nepal, India, and Africa. While we ponder the numerous quality of life issues women experiencing POP navigate, the women in developing zones have additional struggles to overcome, such as little access to adequate healthcare or qualified surgeons. A new medical device, NeuGuide, may provide solutions for the women navigating uterine prolapse in developing and developed zones alike.
Research plays a pivotal role in moving healthcare forward, enabling us to recognize and explore issues common and recognized, or rare and cryptic. Health research has tremendous value for society, providing evidence of health trends, risk factors, treatment outcomes, treatment patterns, and health care costs. Patient voice is being recognized more and more as playing a fundamental role in research development.
Healthcare today advances rapidly, and often it is difficult to funnel sufficient patient numbers into study sites to validate medical innovations. Efficient sharing of information between institution, clinician, and research sectors is pivotal to move the needle forward. The relatively recent medical innovation NeuGuide, a medical device developed at POP Medical Solutions Ltd., an Israeli company founded by Menahem Neuman M.D. and engineer Boaz Harari, is an effort to treat uterine prolapse without removing a healthy but prolapsed uterus. “I’ve spent the last 25 years treating patients with uterine prolapse,” POP Medical founder Dr. Menahem Neuman informed ISRAEL21c. “We don’t want to take out the uterus anymore, because it’s a mistake to take out a healthy uterus.”
NeuGuide for uterine prolapse treatment
Between the years 2012-2015, POP Medical developed their device, conducted pre-clinical studies in both animals and cadavers, and then moved forward with a First-in-Woman Study including 15 patients in Israel. FDA Clearance through the 510K pathway was achieved in late 2016, and in 2017, funds were raised, and IRB approval acquired, for the post-marketing study. POP Medical recently received the CE Mark certification as well, the process required for approval to market in the European Union. In January of 2018, POP Medical began study enrollment.
Since 1998 Vincent Lucente, MD, a urogynecologist based in Allentown, Pennsylvania, has performed several urogynecologic firsts in the United States which have now become “gold standards” of care. Dr. Lucente, one of principle investigators at the 6 sites engaged in the POP Medical research project, took a bit of time out of his hectic schedule to address a few questions regarding uterine prolapse, NeuGuide, and the research surrounding it.
Do you find uterine prolapse to be more or less common now than it was when you began your urogynecologic practice, and if it is more common, do you feel the popularity of marathon running and jogging have played a role increasing the prevalence of POP?
“Given the variations on defining uterine prolapse as a medical condition in terms of both severity (degree of descent) and symptoms, the true incidence rate or prevalence of POP is difficulty to accurately quantify. I am convinced that women are living longer and doing much more during these postmenopausal years than ever before. These activities can range from physically intense recreation such as long distance running, prolonged work years at physically demanding occupations, or care giving to elderly parents or even husbands with impaired mobility. There has no doubt been a shift in expectation of what “life” should involve as the cultural trend of “60 is the new 40” has swept our nation. There has also been a change in women’s willingness to discuss their prolapse symptoms. Today’s more educated and empowered women are no longer accepting of a “suffer in silence” or “just grin and bear it…” approach, they are seeking better answers and treatments to relieve them of their prolapse condition so they may enjoy a better quality of like. Lastly, there continues to be various increasing “pressures” to achieve a vaginal delivery with patients, as such the second stage of labor (time from fully dilated until delivery i.e. the “pushing part”) continues to be “extended”, which has been shown to increase a women’s risk to develop POP. As a result of all of the factors, I notice more and more women coming forward for evaluation and treatment of their POP now, than at the beginning of my career nearly 30 years ago.”
Uterine sparing procedures seem to have become a more popular choice for women navigating uterine prolapse within APOPS' following over the past few years, as opposed to hysterectomy. When a patient comes to you with uterine prolapse, what options do you offer her for surgical treatment, and what considerations influence the options?
“Since the primary supportive defect that results in uterovaginal prolapse has to do with disruption of the connective tissue (ligament like) structures, which essentially attach the vagina to the pelvis, simply removing the uterus will not truly correct the prolapse. In fact, with a vaginal approach to correcting POP utilizing mesh augmented reconstructive surgery, which is my preference, I rarely perform a hysterectomy. I generally only recommend a hysterectomy if it is otherwise indicated (e.g. abnormal bleeding or cervical dysplasia), if the uterus/cervix is the predominant aspect or worse part of the prolapse, or if the cervix itself is extremely elongated or enlarged.
When deciding on mesh reconstruction versus native tissue repair, I look at a patient's risk factors for recurrence. If a patient does not have significant risk factors for recurrence, I would consider a native tissue repair with a laparoscopic uterosacral ligament suspension. I tend to perform this procedure in women of reproductive age, who have a less severe grade of prolapse. Regardless of age, if women have multiple risk factors for recurrence, I would recommend mesh augmentation via a vaginal approach. This procedure is safe when performed by surgeons who are true experts in the technique, it can also be done under regional anesthesia (epidural). Patients often report minimal pain and faster return to recovery because of the vaginal approach.
If a woman is not sexually active, I would consider an obliterative procedure, which means shortening the vaginal length only to about 2-3cm. This procedure takes less time than a reconstructive procedure, and the patients can often go home the same day. It is an ideal procedure for women who are older with multiple medical comorbidities and/or who are not sexually active.”
What interested you about participating in the POP Medical NeuGuide device study?
“The NeuGuide is a novel device that mimics a sacrospinous ligament fixation. The difference is you don't need to do a full dissection into the paravaginal space. Instead, much like a pudendal block, you identify the ischial spine and palpate the sacrospinous ligament through the vaginal epithelium, and simply anchor the vagina to the ligament that way. You then make a small incision in the middle of the vagina to anchor the stitch to the cervix. The potential advantage of this procedure is lower morbidity, lower cost, and faster return to recovery.”
What role do you feel uterine anchoring systems have to play in the future of POP surgical treatment?
“For a select population who has predominantly uterine prolapse (detachment defect), no lateral support defects, no significant risk factors for recurrence, no incontinence, and no voiding dysfunction, this may be an ideal first-line treatment. As you can see the population would be rather restricted, but again, since women are more proactive about reporting symptoms, we may be able to capture this population while they still have a fairly small defect.”
How does the recovery from this procedure compare to other uterine prolapse surgical procedures?
“This procedure would require significantly less recovery time. The incision size and amount of dissection and suturing required would be equivalent to a vaginal delivery with a small laceration. For most major pelvic reconstructive surgeries, we admit patients overnight for observation overnight, but this procedure has the potential to be an ambulatory procedure.”
Patients who engage in research toward the evolution of POP treatment assist with the advancement of healthcare for all women. Some of the reasons to consider participating in a research study are:
- You may have access to new medications or procedures before they are widely available.
- You will have access to physicians without having to wait months for an appointment.
- You contribute to the well-being of society, and the advancement of medical research.
The NeuGuide study is currently recruiting women with uterine prolapse between the ages of 40-85 at the following locations, and would like to capture data from approximately 60 women for this study. Additional study criteria can be viewed at the following ClinicalTrials.Gov web page for The NeuGuide™ System for Vaginal Colpopexy in the Treatment of Uterine Prolapse below:
https://www.clinicaltrials.gov/ct2/show/NCT03436979?term=neuguide&rank=1
Study Locations Recruiting
United States, District of Columbia
MedStar Health Research Institute
Washington, District of Columbia, United States, 20010
Contact: Patricia Tanjutco, M.D. 202-877-5811 Patricia.Tanjuctco@medstar.net
Principal Investigator: Andrew I Sokol, M.D.
Sub-Investigator: Robert E Gutman, M.D.
Sub-Investigator: Cheryl B Iglesia, M.D.
Sub-Investigator: Amy Josphine Park, M.D.
Sub-Investigator: Lee Ann Richter, M.D.
United States, Florida
Cleveland Clinic Florida
Weston, Florida, United States, 33331
Contact: Calvin Killingbeck 954-659-6247 killinc@ccf.org
Principal Investigator: Eric Hurtado, M.D.
Sub-Investigator: Jeffrey Schachar, M.D.
United States, New York
South Nassau Community Hospital Cancer Center
Valley Stream, New York, United States, 11580
Contact: Evelyn Surillo 516-632-3312 Surillo@snch.org
Principal Investigator: Alan Garely, M.D.
Sub-Investigator: Salma Rahimi, M.D.
United States, Pennsylvania
The Institute for Female Pelvic Medicine and Reconstructive Surgery
Allentown, Pennsylvania, United States, 18103
Contact: Eileen Taff 610-730-2502 etaff@fpminstitute.com
Principal Investigator: Vincent Lucente, M.D.
Sub-Investigator: Miles Murphy, M.D.
Sub-Investigator: Jessica Bao An Ton, M.D.
Sub-Investigator: Valerie Schissler, M.S.N., C.R.N.P.
United States, Texas
Walnut Hill OB/GYN Associates
Dallas, Texas, United States, 75231
Contact: John D Bertrand, MD 214-363-7801
Contact: Lora Gantt 214-363-7801 lorapaige@hotmail.com
United States, Virginia
INOVA Women's Hospital
Falls Church, Virginia, United States, 22046
Contact: Rashmi Mishra, M.B.B.S. 703-776-7140 Rashmi.Mishra@inova.org
Principal Investigator: S. Abbas Shobieri, M.D.